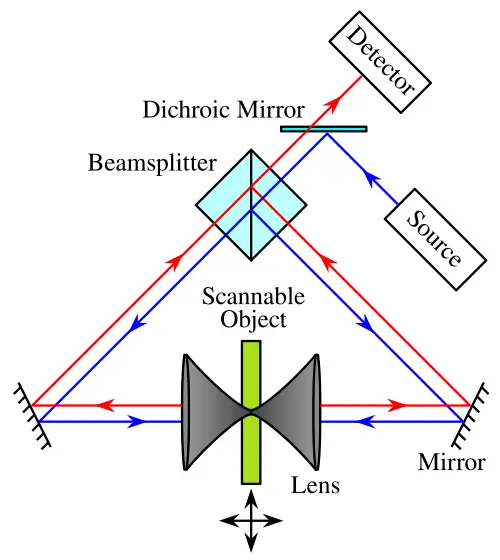
Introduction
Confocal microscopy, especially laser scanning confocal microscopy (LSCM), remains a cornerstone technique in biological, materials, and biomedical research for its ability to produce optically sectioned, high-contrast fluorescence images. However, despite decades of optimization, it still faces challenges: diffraction-limited resolution, photobleaching/phototoxicity, slow acquisition speeds, and noise in low light conditions.
Recently, a surge of new research has focused on combining machine learning (ML), deep learning, and computational methods with confocal imaging to overcome some of these limitations. In this blog, we review major recent advances in AI-driven image enhancement, super-resolution reconstructions, and smarter workflows that are transforming confocal microscopy in 2025 and beyond.
We also discuss the implications for live-cell imaging, throughput, and future directions.
Why image reconstruction and AI are trending in confocal microscopy
Here are key drivers behind this trend:
Low photon budget / phototoxicity concerns: Researchers want to minimize laser power and exposure time to reduce damage to live samples, but this results in noisy, under-sampled data. AI methods can help compensate.Read more
Speed vs. resolution tradeoffs: Faster imaging often sacrifices resolution or contrast. Deep learning can reconstruct high-quality images from faster, lower quality scans.
Multiplexing and multi-channel imaging: As more fluorescent probes and reporters are used simultaneously, separating signal overlap, denoising, and improving resolution becomes harder; computational methods help.
Automation and high throughput: Large datasets from many samples require automated, robust reconstruction and analysis pipelines; AI helps scale this.
Hardware cost and accessibility: Instead of building new, exotic hardware, many of these improvements can be applied via software, making advanced performance more accessible.
These motivations are echoed in recent market analyses: integration of AI and machine learning is seen as a key growth driver in the confocal microscopy market.
Key Recent Advances
Below are some of the most promising and recent trends in computational/confocal microscopy in 2025, along with scientific detail:
1. Physics-informed deep autoencoders for CLSM (Confocal Laser Scanning Microscopy)
A recent work (“Enhanced Confocal Laser Scanning Microscopy with Adaptive Physics Informed Deep Autoencoders”) proposes embedding the PSF (point spread function) and noise models (photon shot noise, dark current, motion blur, etc.) directly into a deep autoencoder architecture. Discover more
Why this is important:
The network learns to “undo” realistic physical degradations in the data, restoring fine structural details even under low light or sparse sampling.
It shows improved metrics (SSIM, PSNR) compared to standard deconvolution or denoising techniques.
It implies faster acquisition, less photodamage, and possibly live imaging with lower laser power.
Challenges and cautions:
Requires accurate modeling of the optical system and noise sources; real-world mismatch may degrade performance.
Generalization: trained models may not work well on very different samples or microscope setups.
Interpretability: deep learning reconstructions may produce artifacts, so validation is critical.
2. Self-supervised learning for multiplex super-resolution confocal microscopy
Another recent advance is in “Self-supervised learning for multiplexing super-resolution confocal microscopy”. Dicover more
What’s new:
This method transforms diffraction-limited confocal images into multi-colour, super-resolution outputs without requiring paired training data.
It uses a degradation model to simulate lower quality input, then trains the network to “undo” that, enabling enhanced resolution and separation of cellular targets without hardware modification.
Demonstrated on both fixed and live cells with two- and three-colour imaging.
Implications:
Makes super-resolution more accessible on standard confocal platforms.
Encourages more widespread multiplex imaging in live biological systems.
Reduces dependency on expensive optical add-ons or specialized hardware.
3. Confocal structured illumination microscopy (SR-CSIM) improvements
Structured illumination is a known method to push resolution beyond the diffraction limit. Recent theoretical and numerical work (“Confocal structured illumination microscopy for super-resolution imaging: theory and numerical simulations”) describes SR-CSIM that integrates structured illumination principles with confocal scanning to improve resolution while reducing artifacts. Read more
Advantages:
Avoids needing to estimate structured illumination parameters (which often introduce errors).
Provides robust resolution gains comparable to SIM but with fewer artifacts.
Potentially more applicable to real samples.
Considerations:
Theory and simulations are promising, but experimental validation on biological samples and complex specimens is needed.
Hardware implementation and alignment may still be non-trivial.
4. Numerical refocusing and synthetic holography in confocal systems
Another emerging area is computational refocusing in coherent confocal laser scanning microscopy, using synthetic holography to digitally refocus images obtained at different offsets. Learn more
Potential benefits:
Enables post-acquisition focusing, which is useful when samples have height variation or are not flat.
Reduces need for mechanical z-scanning or precise focusing during imaging.
May improve throughput and robustness, especially in complex 3D samples.
Limitations:
Requires coherent detection and phase information, which may not always be available in standard fluorescence systems.
Sensitivity to aberrations and noise; real biological samples may pose challenges.
5. Advances in hardware and novel imaging modalities supporting confocal improvements
Beyond pure software, related trends in hardware and system design are enhancing confocal performance:
Adaptive optics and three-photon microscopy: Three-photon adaptive optics microscopy (3PAOM) allows deep tissue imaging with correction for aberrations, enabling clearer confocal-like imaging deeper in scattering media. Discover more
Structured illumination and light-sheet hybrid methods: Techniques like structured illumination light sheet microscopy (SI-LSM) and lattice light-sheet are pushing 3D imaging speed and reducing photodamage, potentially complementary to confocal. Discover more on wikipedia
Vertico spatially modulated illumination: Modulating illumination patterns (e.g. via SMI) to engineer PSF and improve resolution or measurement precision.Learn more
4Pi and dual-objective confocal enhancements: Improving axial resolution through coherent illumination and detection from both sides of sample. Wikipédia
Applications and Impacts
These computational and hardware advances are driving new opportunities and impacts in confocal microscopy:
Live-cell super-resolution imaging: Achieving higher spatial and temporal resolution with less phototoxicity, enabling better tracking of dynamic processes (organelle interactions, cytoskeletal changes, signaling).
High throughput screening: Automating image enhancement and analysis using AI allows screening many samples (drug libraries, genetic perturbations) faster and with better quality.
Thick tissue and 3D culture imaging: Improved refocusing, denoising, and deep-tissue imaging help in studying organoids, neural tissues, and developmental biology.
Accessibility of super-resolution tools: By embedding enhancements via software, more labs with standard confocal setups can perform advanced imaging without needing the latest hardware.
Reduced sample preparation burden: Better reconstructions may reduce the need for high fluorophore concentrations, strong signal amplification, or aggressive clearing, preserving sample integrity.
Live Cell Imaging
High throughput screening
Fluorescence Colocalization Studies
High-Resolution Imaging of Subcellular Structures
Challenges, Limitations, and Considerations
To maintain scientific rigor, it's important to recognize the following caveats and challenges:
Validation and reproducibility: AI-based reconstructions may generate features that are artifacts rather than real structures. Independent validation remains critical.
Generalization across setups and samples: Models trained on one microscope/optics/sample type may not translate well to others. Transfer learning and calibration are required.
Computational cost and resource requirements: High-quality deep learning models demand substantial GPU/CPU resources, storage, and expertise.
Integration with existing workflows: Adapting algorithms to the software ecosystems of different microscopy platforms is nontrivial.
Interpretation and bias risk: Using computational enhancements can introduce unintended biases in quantitative measurements; transparency and documentation are necessary.
Future Outlook and Directions
Here are some promising directions for the future (2025–2030) in confocal and related microscopy:
Hybrid AI-hardware co-designed systems: Designing microscope optics and detection systems in tandem with AI reconstruction to optimize overall performance.
Real-time reconstruction and feedback: On-the-fly AI enhancement allowing “smart” imaging that adapts scanning parameters based on sample feedback.
Multimodal integration: Combining confocal imaging with other modalities (e.g. electron microscopy, Raman, expansion microscopy) and reconstructing correlative images via AI.
Better transfer learning and generalizable models: Creating “universal” denoising/enhancement models that adapt to varying instruments and sample types.
Open datasets and benchmarking standards: Developing shared datasets, benchmarks, and metrics for comparing computational methods in microscopy.
Conclusion
In summary, the integration of AI, deep learning, and computational techniques with confocal microscopy is a major emerging trend, reshaping what's possible in terms of resolution, speed, noise reduction, and accessibility. The recent advances in physics-informed autoencoders, self-supervised super-resolution, and structured illumination reconstructions illustrate a strong movement toward software-based performance improvements. When coupled with hardware innovations such as adaptive optics, three-photon excitation, and structured illumination, the future of confocal imaging looks richer, more powerful, and more accessible than ever.